The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor

FFUL LisbonHilde Boone 29 May 2003 EMEA 1 Implementation of the CTD in Europe & EMEA Experiences Evaluation and Regulation of Medicines & Health Products. - ppt download
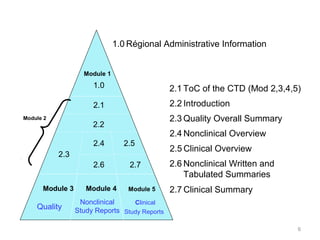
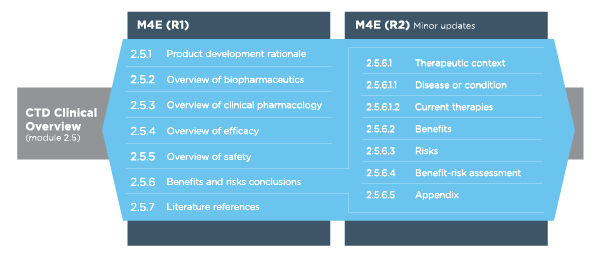
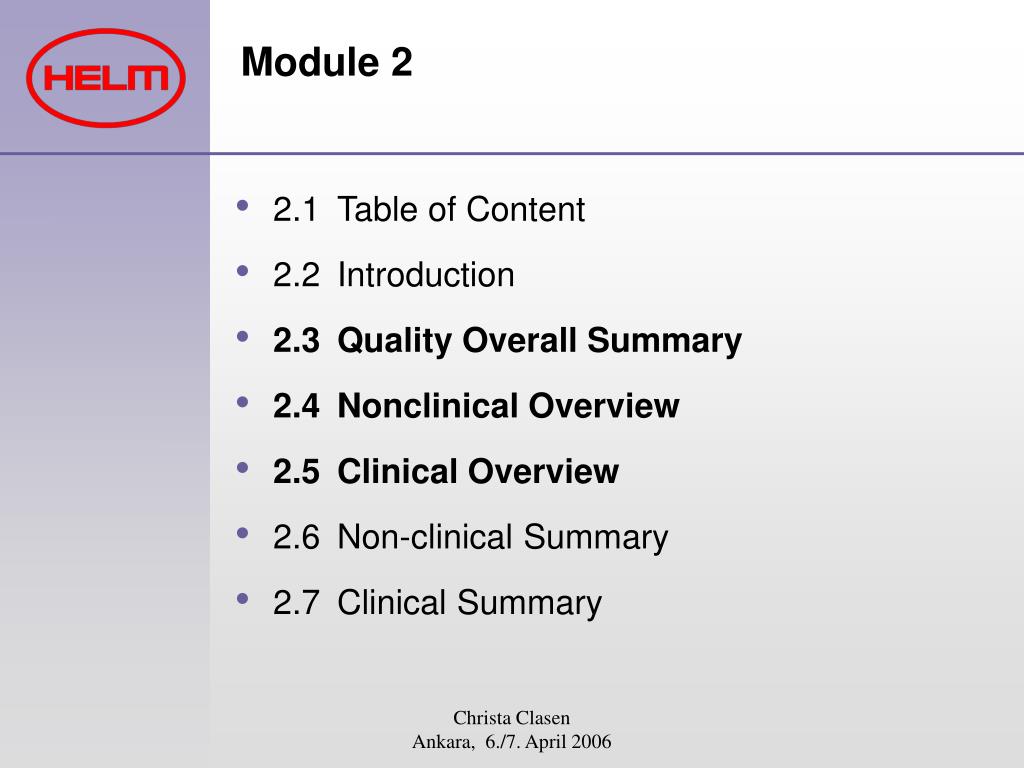
Clinical Study Reports Quality 2.7 Clinical Summaries 2.5 Clinical Overview 2.3 Quality Overall Summary 22.6 Non-Clinical Summar

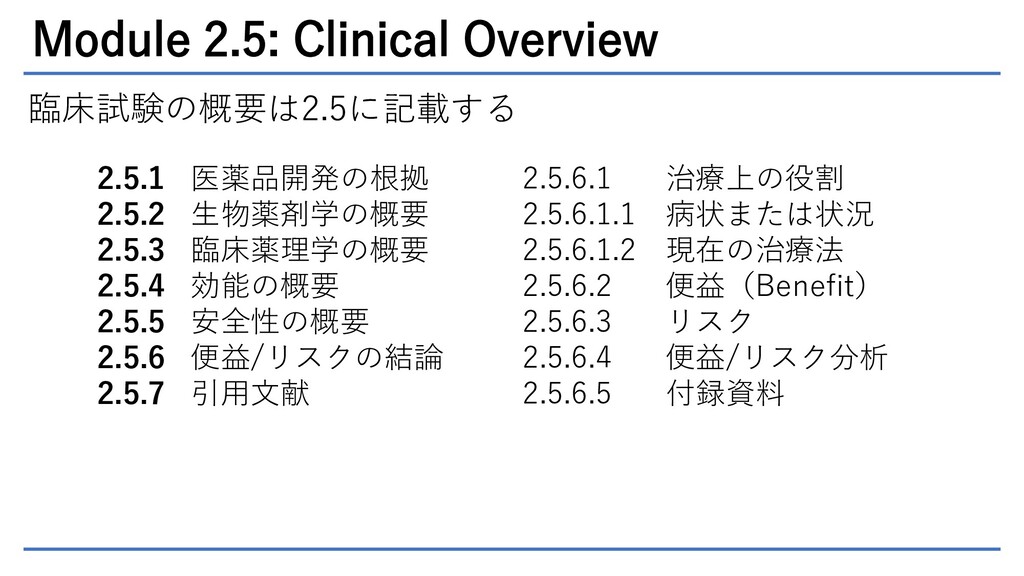
TuraSkills shares tip for writing #Module 2.5 #Section 2.5.2 #Overview of Biopharmaceutics #Clinical overview #CTD overvie… | Writing tips, Marketing data, Writing