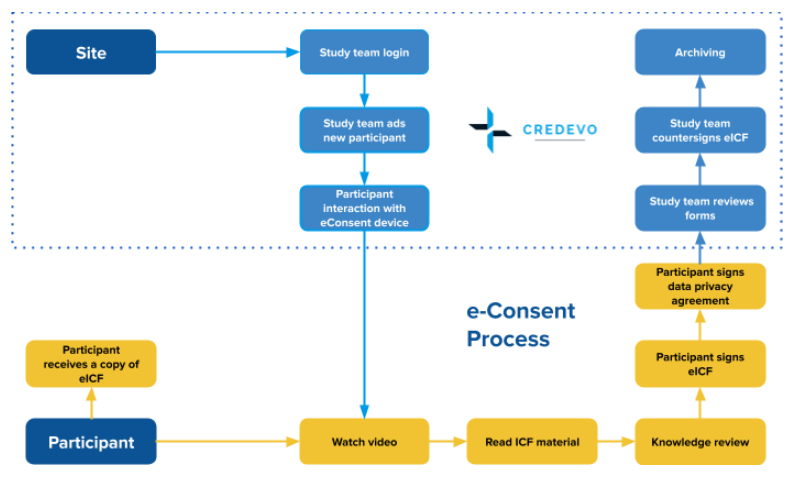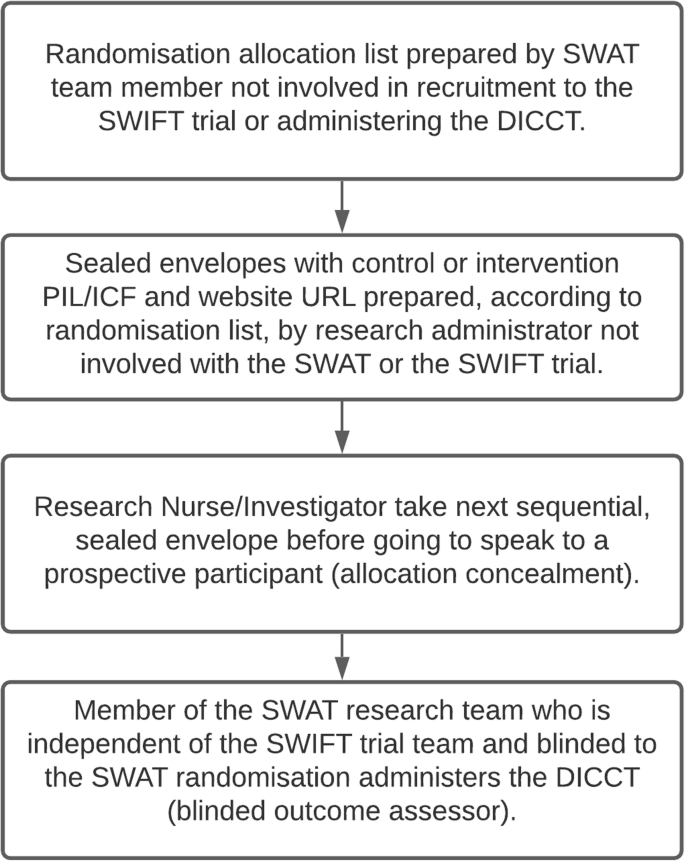
An enhanced participant information leaflet and multimedia intervention to improve the quality of informed consent to a randomised clinical trial enrolling people living with HIV and obesity: a protocol for a Study

Potential Risks and Mitigation Strategies Before the Conduct of a Clinical Trial: An Industry Perspective | Bentham Science
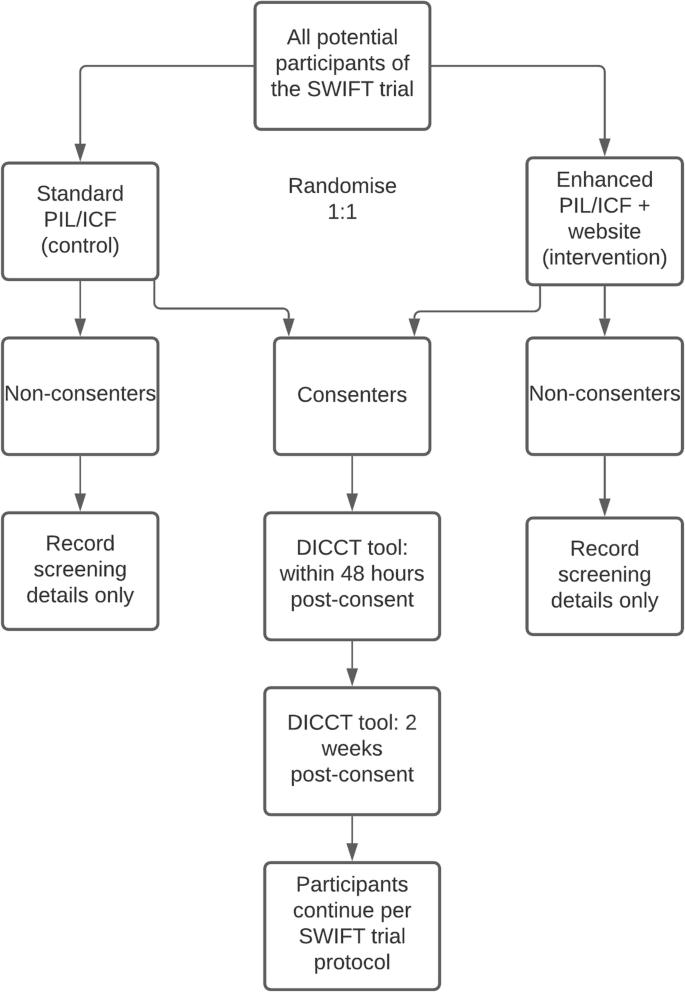
An enhanced participant information leaflet and multimedia intervention to improve the quality of informed consent to a randomised clinical trial enrolling people living with HIV and obesity: a protocol for a Study

PDF) Project Management of Randomized Clinical Trials: A Narrative Review | Hamidreza Goodarzynejad - Academia.edu
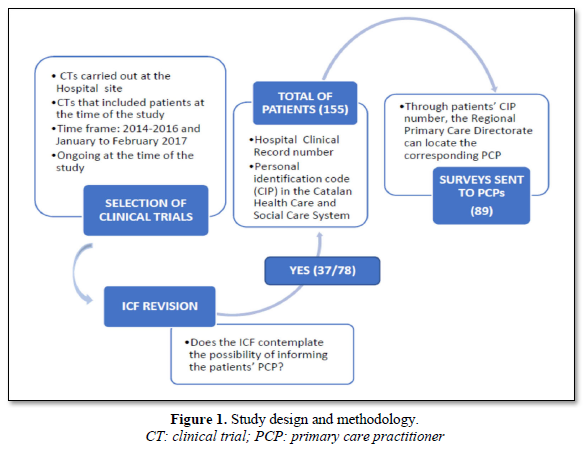
SCITECH - Informed Consent Procedure in Clinical Trials Promoted by the Hospital: Knowledge and Perceptions of Primary Care Physicians - Journal of Clinical Trials and Research (ISSN:2637-7373)