Overview of comments - Requirements to the chemical and pharmaceutical quality documentation concerning investigational medicina
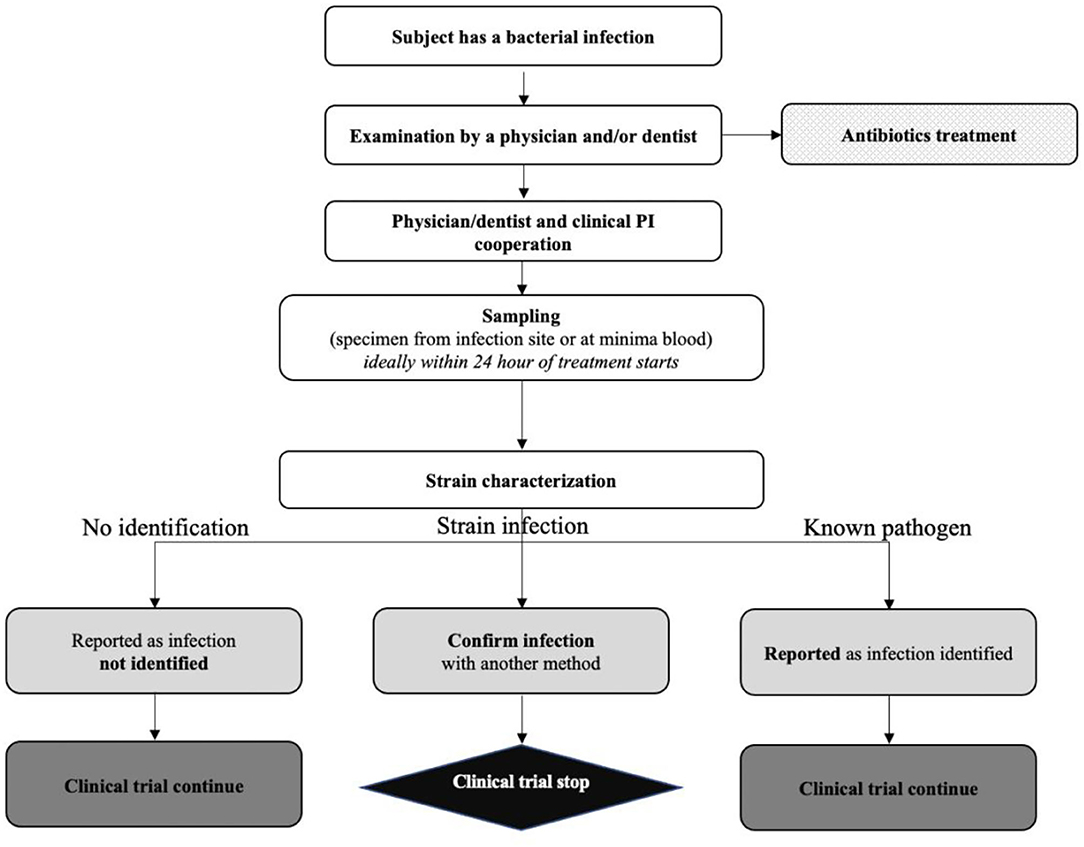
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA

Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy
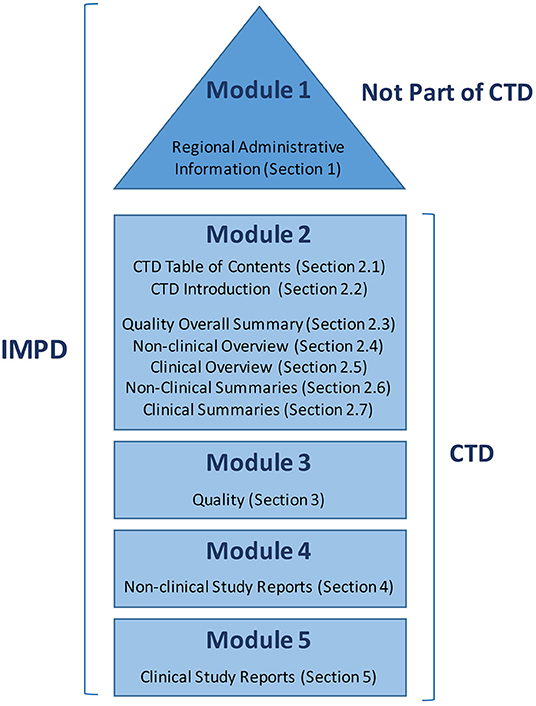
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience
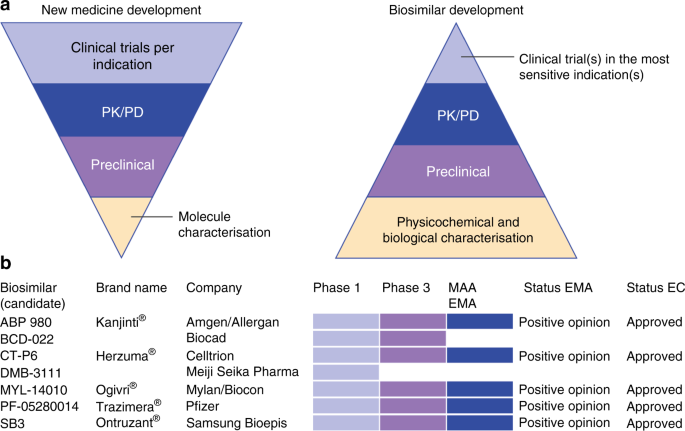
The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars | British Journal of Cancer
Reflection paper on guidance for laboratories that perform the analysis or evaluation of clinical trial samples












