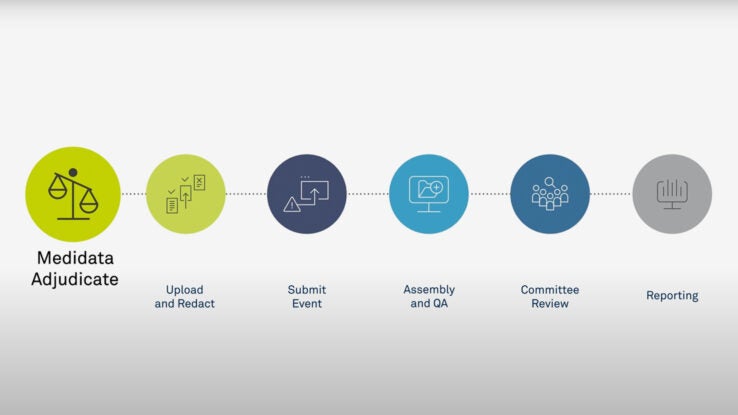
2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee

Clinical-event committee adjudicated endpoint rates. The figure report... | Download Scientific Diagram
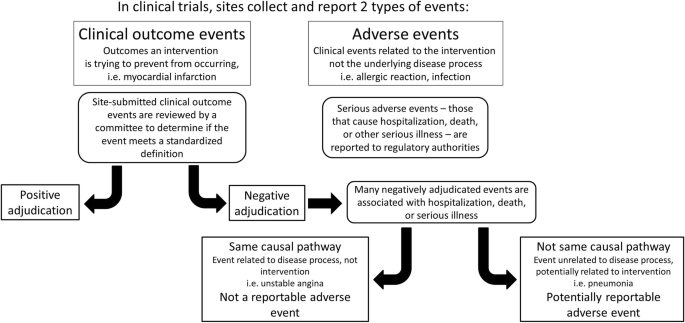
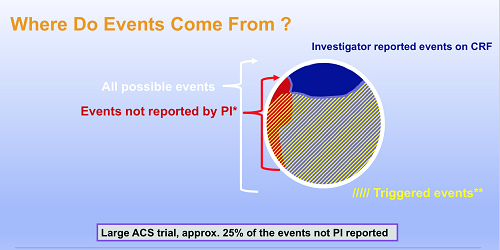
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text

Central Adjudication Identified Additional and Prognostically Important Myocardial Infarctions in Patients Undergoing Percutaneous Coronary Intervention | Circulation: Cardiovascular Interventions

Clinical events classification (CEC) in clinical trials: Report on the current landscape and future directions — proceedings from the CEC Summit 2018 - ScienceDirect

GLASSY design. CEC, Clinical Event Committee; GLASSY, GLOBAL LEADERS... | Download Scientific Diagram
Inadequate planning and reporting of adjudication committees in clinical trials: Recommendation proposal

Vascular Complications after Transfemoral Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis - Structural Heart